Vero Cell Vaccine Covid-19 Efficiency / Sars Cov 2 Mutations Vaccines And Immunity Implication Of Variants Of Concern Signal Transduction And Targeted Therapy
NVX-CoV2373 developed by Novavax rolling review started on 3 February 2021 CVnCoV by Curevac rolling review started on 12 February 2021 Sputnik V Gam-COVID-Vac by Gamaleya rolling review started on 4 March 2021 and COVID-19 Vaccine Vero Cell Inactivated by Sinovac Life Sciences Co Ltd rolling review started. Participants will be randomly inoculated with two doses of investigational vaccine or placebo according to 11 ratio following Day 0-Day 14 immunization schedule and will be observed from the first dose of investigational vaccine to collect symptomatic and laboratory-confirmed COVID-19 cases for the evaluation of the efficacy of the investigational vaccine.

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
There is no substantial data showing how protective Johnson Johnsons single-dose COVID-19 vaccine is and US.

Vero cell vaccine covid-19 efficiency. Its true that the Vero cells are very popular. However this vaccine is an inactivated vaccine with an adjuvant that is routinely used in many other vaccines with a documented good safety profile including in pregnant women. With a correlation efficiency.
As 1 July 2021 six of the 71 COVID-19 deaths in Seychelles were among the fully vaccinated people. So far Vero is the only Chinese vaccine for which the manufacturer has published official data. A large phase 3 trial in Brazil showed that two doses administered at an interval of 14 days had an efficacy of 51 against symptomatic SARS-CoV-2 infection 100 against severe COVID-19 and 100 against hospitalization starting 14 days after receiving the second dose.
EMA has initiated rolling reviews for the following COVID19 vaccines. At the start date. Especially in times like the current COVID-19 pandemic advanced and scalable platform technologies could provide more efficient and cost-effective solutions to meet the global vaccine demand.
Estimating vaccine efficacy for COVID-19 projections Currently the IHME model uses the following inputs of vaccine efficacy separated by variant. When a person is given the vaccine their immune system identifies the inactivated virus as foreign and makes antibodies against it. The reporter neutralization assay should be useful for high-throughput evaluation of COVID-19 vaccines.
Herein we review the prevailing literature on Vero cell bioprocess development for the production of viral vectors and vaccines with the aim to assess the recent advances in bioprocess development. The Sinopharm COVID-19 vaccine vero cell inactivated vaccine resource includes key information on the vaccine specific requirements. COVID-19 Vaccine Vero Cell Inactivated CoronaVac COVD1 Vaccine Explainer 11 JUNE 1 Recommended for age 18 to 59 years of age as per WHO EUL Based on the data reviewed WHO SAGE recommends use in persons aged 18 years and above.
This is operationalized as a reduction in hospitalization and death. COVID-19 Vaccine Vero Cell Inactivated also contains an adjuvant a substance that helps strengthen the immune response to the vaccine. Vaccine Efficacy 8350 CI 95 6542-9212 Based on symptomatic and RT-PCR positive COVID-19 cases after 14 days and more after the 2nd dose Number of subjects after 14 days and more after the 2nd dose Based on calculation person x year in the follow up period Treatment Arm Number of hospitalized COVID-19 Cases Total Subject Number Person year.
A Study to Evaluate the Efficacy Safety and Immunogenicity of SARS-CoV-2 Vaccine Vero Cells Inactivated in Healthy Adults Aged 18 Years and Older COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Recommended schedule 2 doses 05 mL each at a recommended interval of 2 to 4 weeks. Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
On December 29 2020 Sinopharm reported 79 efficacy in an interim evaluation. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. The available data on the COVID-19 vaccine BIBP in pregnant women are insufficient to assess either vaccine efficacy or vaccine-associated risks in pregnancy.
But unfortunately for this particular aspect of Covid-19 research they are absolutely not useful. In December 2020 UAEs Ministry of Health and Prevention previously announced interim analysis showing BBIBP-CorV to have a 86 efficacy against COVID-19 infection and nearly 100 efficacy in preventing moderate and severe cases. I think this is now clear to the field.
Efficacy at preventing symptomatic disease. Vacina contra a COVID-19 Vero CellInactivada CoronaVac. After incubation at 37C for 1 h Vero CC-81 cells.
Infectious disease experts are weighing the need for.
Expert Chinese Vaccines Effective Against Covid 19 Delta Variant Cgtn

Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News

Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
500 000 Doses Of Sinopharm Covid 19 Vaccine Arrive In Vietnam Today

Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says

Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use
World Health Organization Who What S The Difference Between Covid 19 Vaccine Efficacy And Effectiveness Vaccine Efficacy Refers To How The Vaccine Performs In Ideal Conditions Controlled Clinical Trials Vaccine
Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace
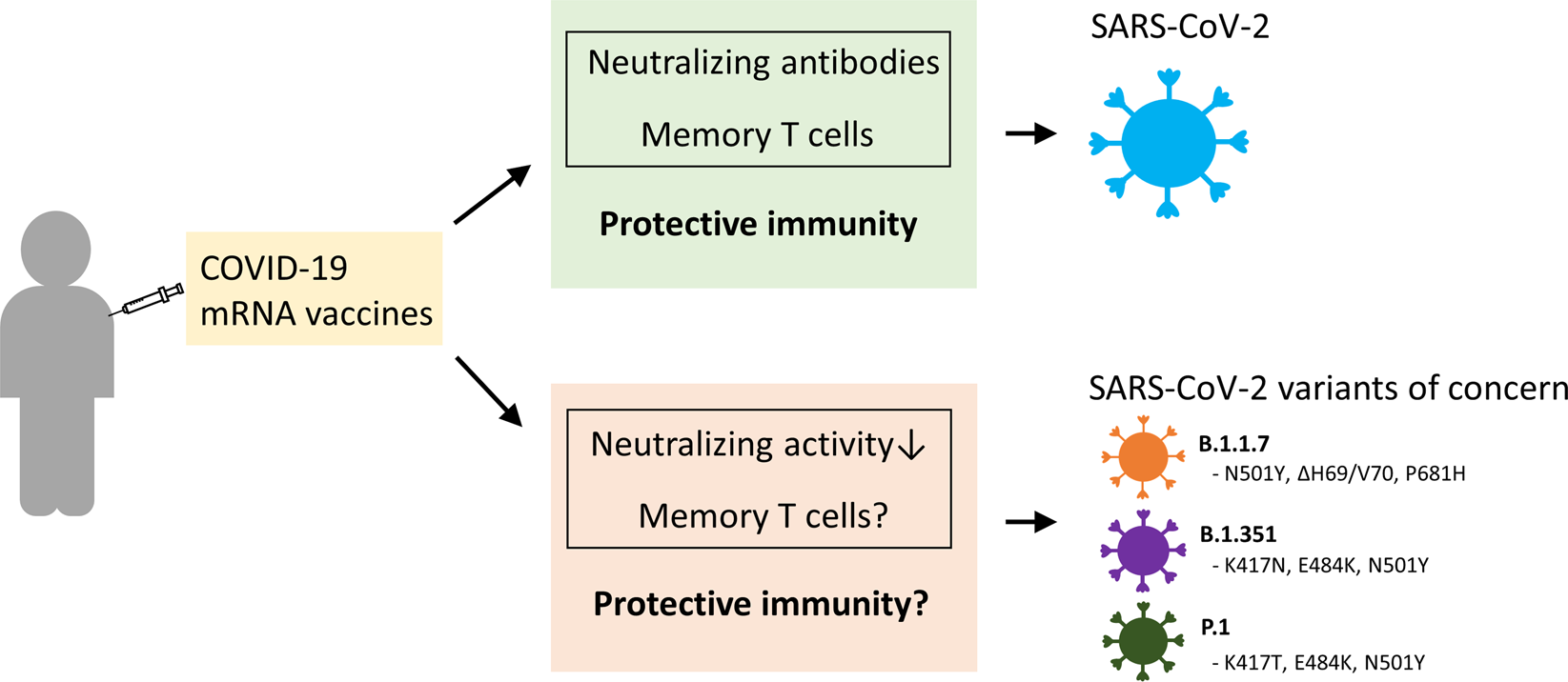
Sars Cov 2 Mutations Vaccines And Immunity Implication Of Variants Of Concern Signal Transduction And Targeted Therapy

Who Approves A Covid 19 Vaccine From China S Sinopharm For Emergency Use
Sinovac Covid 19 Vaccine Safe For Ages 3 To 17 In Small Early Trial Devex
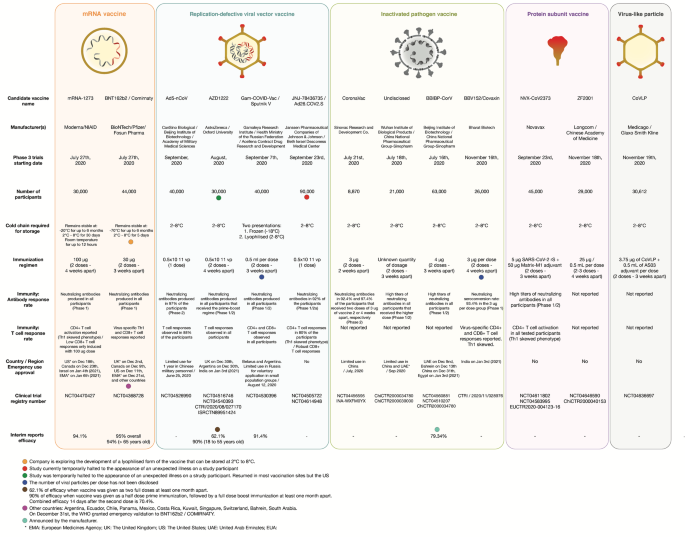
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines
Doubts Over China Vaccines Effectiveness Mar Production Push Nikkei Asia