Vero Cell Vaccine Covid-19 - Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews
COVID-19 Vaccine Vero Cell Inactivated Sinopharm COVID-19 Vaccine Explainer 24 MAY 2021 Special population groups based on available data as of 7 May 2021 For persons with comorbidities phase 3 clinical trial data are insufficient to determine vaccine efficacy. 2 clinical efficacy and safety.
Singapore Excludes Sinovac Shots From Covid 19 Vaccination Tally Nikkei Asia
Its easy storage requirements make it highly suitable.
Vero cell vaccine covid-19. As 1 July 2021 six of the 71 COVID-19 deaths in Seychelles were among. The SARS-CoV-2 Vaccine VeroCell is an inactivated vaccine against coronavirus disease 2019 COVID-19 which stimulates the bodys immune system without risk of causing disease. Vero cell vaccine covid-19.
The vaccine contains SARS-CoV-2 that has been inactivated killed and cannot cause the disease. So far Vero is the only Chinese vaccine for which the manufacturer has published. If anything the vaccine choice has added to the complication.
COVID-19 Vaccine Vero Cell Inactivated COMPOSITION Active ingredient. On December 29 2020 Sinopharm reported 79. Disodium hydrogen phosphate dodecahydra te sodium dihydrogen phosphate monohydrate sodium chloride DESCRIPTION CoronaVac is a milky-white suspension.
Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Once the inactivated vaccine gets presented. According to the notice published by authorities Vero cell administration drive this time will include all people aged above 55 who have yet to receive their first dose University professors.
The governments decision to administer the Vero Cell vaccine developed by Chinas Sinopharm is not the right choice of vaccine for migrant workers stakeholders say. Vero cell vaccine sinopharm or sinovac. COVID-19 Vaccine Vero Cell Inactivated Brief Edition.
Pursuant to the role of the Health Technology Assessment Council HTAC to develop coverage recommendations particularly in the selection and financing of COVID-19 vaccines using the Evaluation Framework set by the HTAC this report. More Chinese-made Covid-19 vaccine doses arrive in HCMC Thanh Ha - August 16 2021 HCMC An additional one million doses of Chinas Vero Cell Covid-19 vaccine part. SARS-CoV-2 Vaccine Vero Cell Inactivated CoronaVac for the prevention of COVID-19 July Updates Date of publication.
Vero cell vaccine covid-19 efficacy. A large multi-country Phase 3 trial has shown that 2 doses administered at an interval of 21 days have an efficacy. Nepal has granted emergency approval to the chinese vero cell vaccine given the active caseload that stands at 277944 on monday while 273240 people have recovered in nepal.
The addition of this vaccine has the potential to rapidly accelerate COVID-19 vaccine access for countries seeking to protect health workers and. COVID-19 Vaccine Vero Cell Inactivated is expected to prepare the body to defend itself against infection with SARS-CoV-2. Sinopharm die zulassung für seinen impfstoff mit dem.
Decision of the Secretary of Health. CoronaVac is an inactivated vaccine to be administered intramuscularly as a course of 2 doses each dose of 05ml contains 600SU. The Sinopharm COVID-19 vaccine vero cell inactivated vaccine resource includes key information on the vaccine specific requirements.
Sinopharm Vero Cell Inactivated Covid 19 Vaccine. On December 29 2020 Sinopharm reported 79 efficacy in an interim evaluation. Once inactivated viruses get presented to the bodys immune system they stimulate the production of.
However this vaccine is an inactivated vaccine with an adjuvant that is commonly used in many other vaccines with a well-documented safety profile such as. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Vero Cell is produced by Beijing Bio-Institute of Biological Products Co Ltd a subsidiary of China National Biotec Group CNBG.
3 affordability and viability. The COVID-19 Vaccine Vero Cell Inactivated CoronaVac is an inactivated vaccine against coronavirus disease 2019 COVID-19 which stimulates the bodys immune system without risk of causing a disease. The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell.
Providing Covid-19 vaccines to outgoing migrants was necessary. Sinopharm Vero Cell Inactivated Covid 19 Vaccine. If anything the vaccine.
The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell. Inactivated SARS-CoV-2 Virus CZ02 s train Adjuvant. The World Health Organization WHO has listed Sinopharms Vero Cell COVID-19 vaccine for emergency use allowing it to be rolled out globally.
So far Vero is the only Chinese vaccine for which the manufacturer has published official data.

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use
What Is Vero Cell Ema Launches Rolling Review Of Chinese Covid 19 Vaccine Euronews

Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
Sinopharm Vaccine Side Effects Price Efficacy Of China S Sinopharm Covid Vaccine India News Times Of India

Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021

European Drugs Regulator Starts Rolling Review Of Sinovac Anti Coronavirus Jab

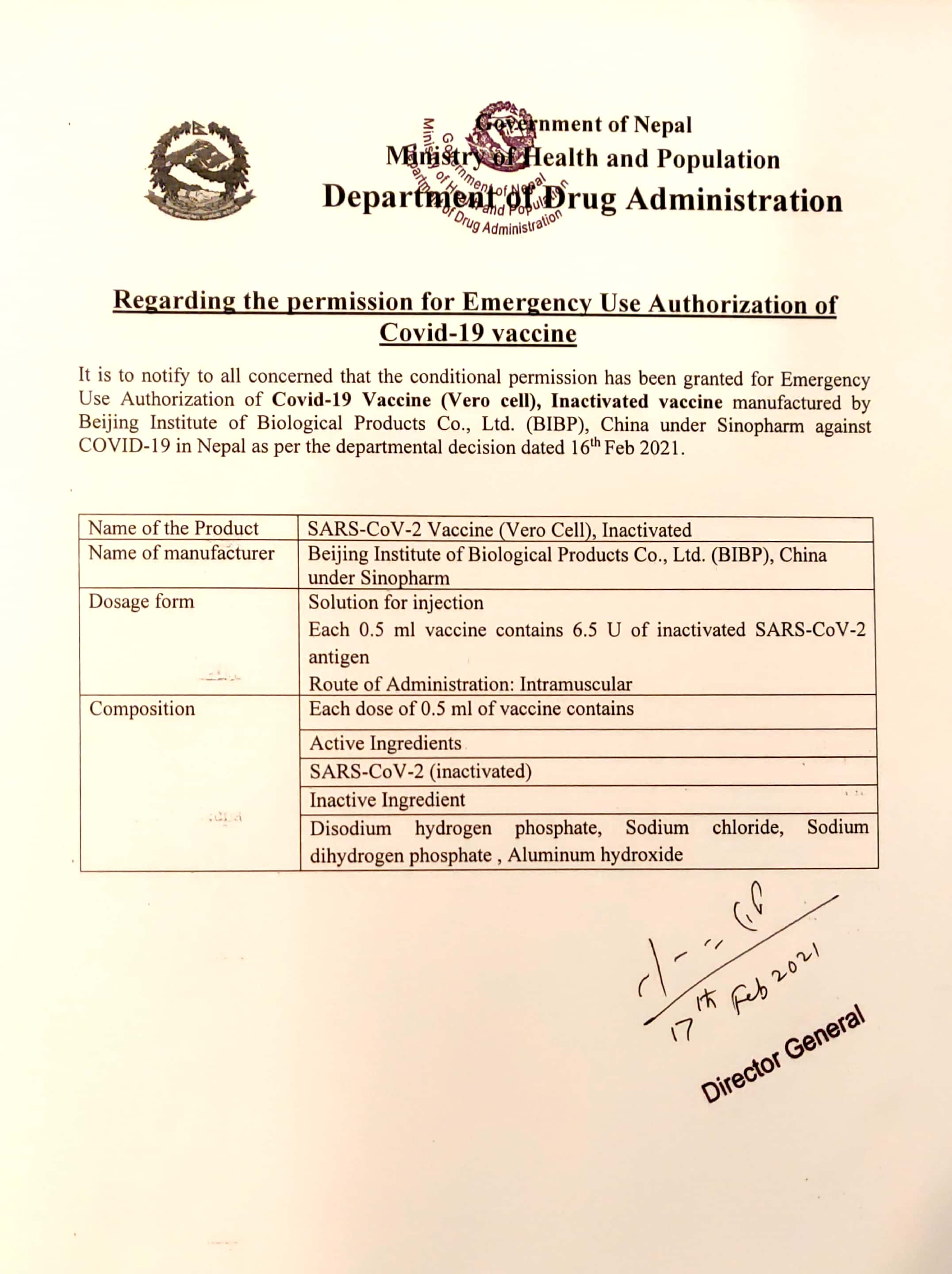
Dda Eua Of Covid 19 Vaccine Vero Cell Inactivated Manufactured By Bibp Under Sinopharm
Egypt Reports No Serious Side Effects On Chinese Covid 19 Vaccine Cgtn
500 000 Doses Of Sinopharm Covid 19 Vaccine Arrive In Vietnam Today

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says

Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

China Grants First Approval Of Homegrown Covid 19 Vaccine Voice Of America English

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News
Sinovac Covid 19 Vaccine Safe For Ages 3 To 17 In Small Early Trial Devex

Who Approves A Covid 19 Vaccine From China S Sinopharm For Emergency Use

Taiwan Puts All Eggs In One Basket By Refusing Mainland Vaccines Global Times
Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace

.JPG)