Vero Cell Vaccine Covid-19 Uae : The Uae Approves A Chinese Made Coronavirus Vaccine For Emergency Use
Sinopharm BBIBP-CorV also known as the Sinopharm COVID-19 vaccine is one of two inactivated virus COVID-19 vaccines developed by Sinopharm. Decision of the Secretary of Health.

Uae Capital Mandates Booster Shot For Sinopharm Covid 19 Vaccine Recipients Business Standard News
SARS-CoV-2 Vaccine Vero Cell Inactivated CoronaVac for the prevention of COVID-19 July Updates Date of publication.

Vero cell vaccine covid-19 uae. COVID-19 Vaccine Vero Cell Inactivated Brief Edition Title. Yes vaccination is safe for older adults and people with chronic conditions. FILE - A box for a COVID-19 vaccine is displayed at.
On Monday the UAE health authorities said the experimental vaccine will be administered to frontline healthcare workers who are dealing with cases of COVID-19 and are at higher risk of exposure. The UAEs National Emergency Crisis and Disaster Management Authority announced the. It is recommended to provide the vaccine to these groups as they are at risk of developing complication when infected with COVID-19.
The efficacy rating followed an interim report of ongoing human trials conducted in that country CNBC reported. The plane with the medication on board arrived in Minsk on 9 June BelTA learned from the Telegram-channel of the Belarusian Healthcare Ministry. BBIBP-CorV also known as the Sinopharm COVID-19 vaccine or BIBP vaccine is one of two inactivated virus COVID-19 vaccines developed by Sinopharms Beijing Institute of Biological Products sometimes written as Beijing Bio-Institute of Biological Products resulting in the two different acronyms BBIBP and BIBP for the same vaccine.
The medical team will evaluate in case there is a reason to prevent getting vaccinated. The COVID-19 vaccine under development by Chinas Sinopharm is showing efficacy of 86 health authorities from the United Arab Emirates reported this morning. Inactivated SARS-CoV-2 Virus CZ02 s train Adjuvant.
A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Around the end of December the COVID-19 Pfizer-BioNTech vaccine was approved to be administered in Dubai. China joined this club of elites of science following the Chinese FDA approval of Sinopharm the subsidiary of state-owned China National Pharmaceutical Group- CNPG first COVID-19 vaccine inactivated Sars-Cov-2 based on the results of the phase-3 clinical trial in UAE and Bahrain showing up to 86 efficacy of the vaccine in preventing COVID-19.
Recommended schedule 2 doses 05 mL each at a recommended interval of 2 to 4 weeks. Kathmandu Nepal April 4 ANI. China-based pharmaceutical company Sinopharm has initiated a Phase III clinical trial to assess its Covid-19 vaccine candidate in Abu Dhabi UAE.
At the start date Dose 2. After undergoing numerous clinical trials the. Sinopharrm initiated a Phase III study of its vaccine candidate in UAE in July Reuters reported.
Vaccine COVID-19 Vero Cell của Sinopharm được Trung Quốc cấp phép ngày 24122020 và Tổ chức Y tế Thế giới WHO phê duyệt khẩn cấp tháng 52021. BBIBP-CorV shares similar technology with CoronaVac and. Việt Nam đã tiếp nhận 500000 liều vaccine Sinopharm do Trung Quốc viện trợ và đang triển khai tiêm chủng từ tháng 72021.
Belarus COVID-19 latest. The candidate is said to be the worlds first inactivated vaccine to enter a Phase III trial. The Sinopharm COVID-19 vaccine vero cell inactivated vaccine resource includes key information on the vaccine specific requirements.
COVID-19 Vaccine Vero Cell Inactivated COMPOSITION Active ingredient. COVID-19 Vaccine Vero Cell Inactivated Brief Edition Created Date. Detail of trials of Sinopharm inactivated COVID-19 vaccines Vero Cells.
COVID-19 Vaccine Vero Cell Inactivated CoronaVac COVD1 Vaccine Explainer 11 JUNE 1 Recommended for age 18 to 59 years of age as per WHO EUL Based on the data reviewed WHO SAGE recommends use in persons aged 18 years and above. Disodium hydrogen phosphate dodecahydra te sodium dihydrogen phosphate monohydrate sodium chloride DESCRIPTION CoronaVac is a milky-white suspension. 14 to 28 days after.
Sputnik V COVID-19 vaccine. The Emirates Health Ministry announced the news Wednesday about the vaccine developed by Chinas state-owned pharmaceutical company Sinopharm. On 21 January 2021 Ministry of Health and Prevention MoHAP announced its approval of Russias Sputnik V COVID-19 vaccine for emergency use in the UAE.
Nepal is set to start the second phase of its COVID-19 inoculation drive using China-made Vero Cell vaccine sans concerns over safety and approval from the. The vaccine is based on human adenoviral vector-based platform. Especially in times like the current COVID-19 pandemic advanced and scalable platform technologies could provide more efficient and cost-effective solutions to meet the global vaccine demand.
It completed Phase III trials in Argentina Bahrain Egypt Morocco Pakistan Peru and the United Arab Emirates UAE with over 60000 participants. Its effectiveness against COVID-19 is confirmed at 914 per cent while its efficacy against severe cases of the disease is 100 per cent. Dec 09 2020 By Alex Keown.
607 new cases 434 recoveries MINSK 9 June BelTA - The United Arab Emirates UAE has donated the vaccine against coronavirus to Belarus. Herein we review the prevailing literature on Vero cell bioprocess development for the production of viral vectors and vaccines with the aim to assess the recent advances in bioprocess development. It completed Phase III trials in Argentina Bahrain.
Pursuant to the role of the Health Technology Assessment Council HTAC to develop coverage recommendations particularly in the selection and financing of COVID-19.

Uae Officially Registers China S Sinopharm S Covid 19 Vaccine Cgtn
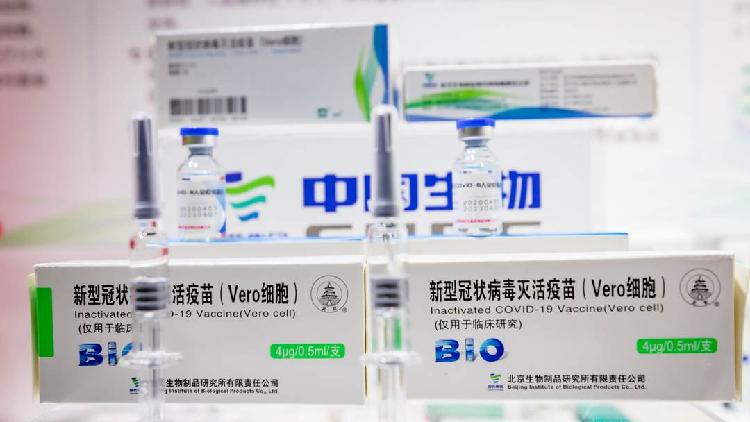
Egypt Reports No Serious Side Effects On Chinese Covid 19 Vaccine Cgtn

Uae First Country In Arab World To Begin Manufacturing Covid 19 Vaccine Mobihealthnews

Vaccine Diplomacy Sees Egypt Roll Out Chinese Coronavirus Jab Global Health The Guardian
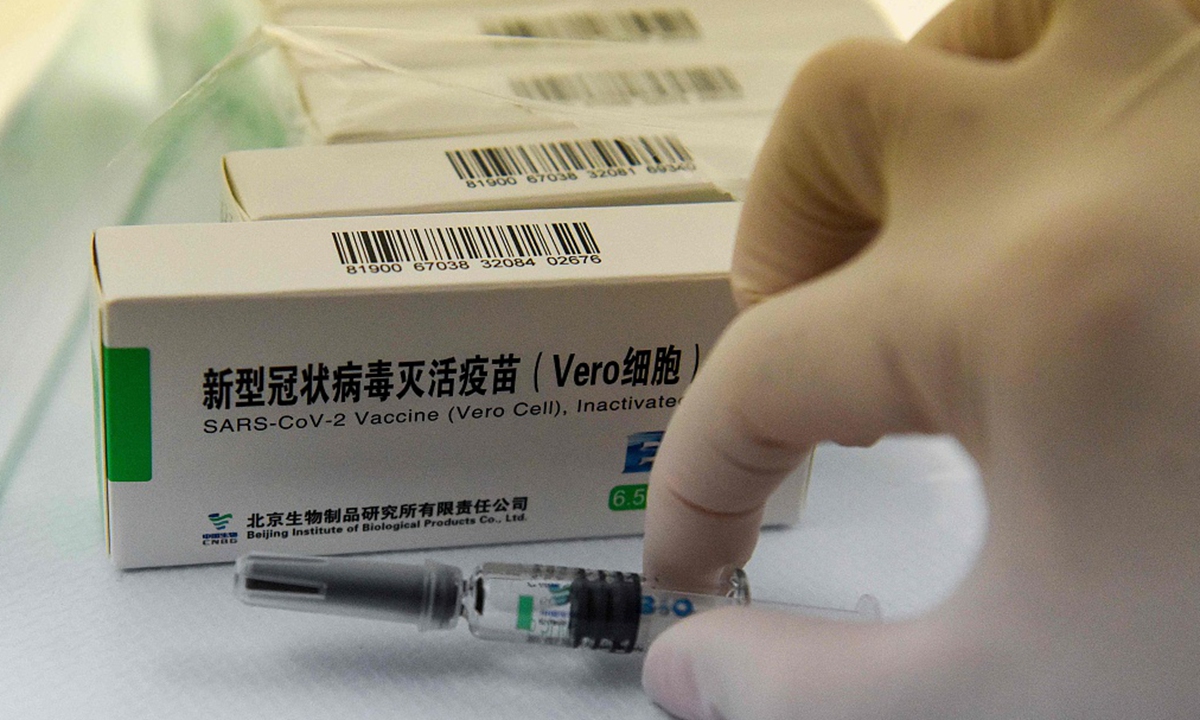
Phase Iii Clinical Trials With 45 000 Participants In Arab Countries Show Efficacy Of Sinopharm Covid 19 Vaccine Is 78 89 On Adults Global Times
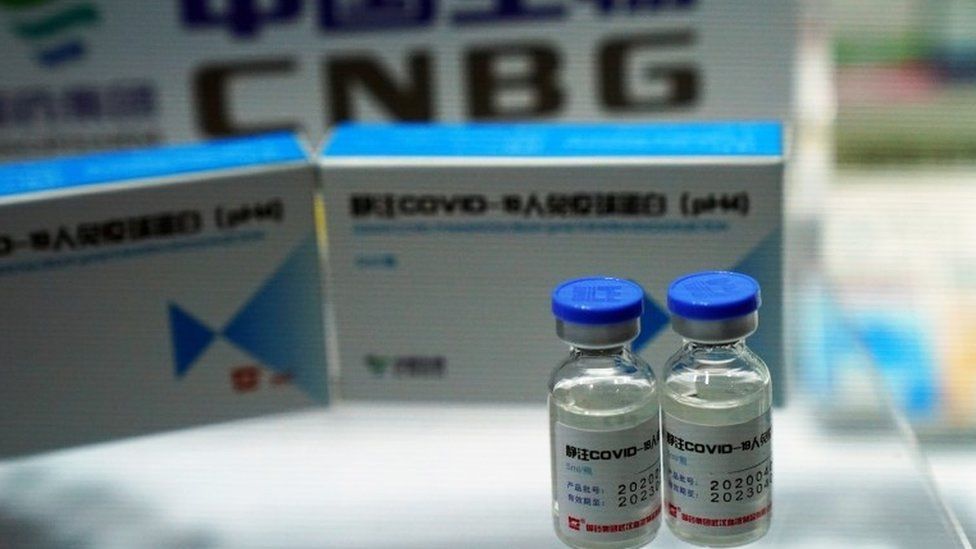
Coronavirus Vaccine China Jab 86 Effective Uae Says Bbc News
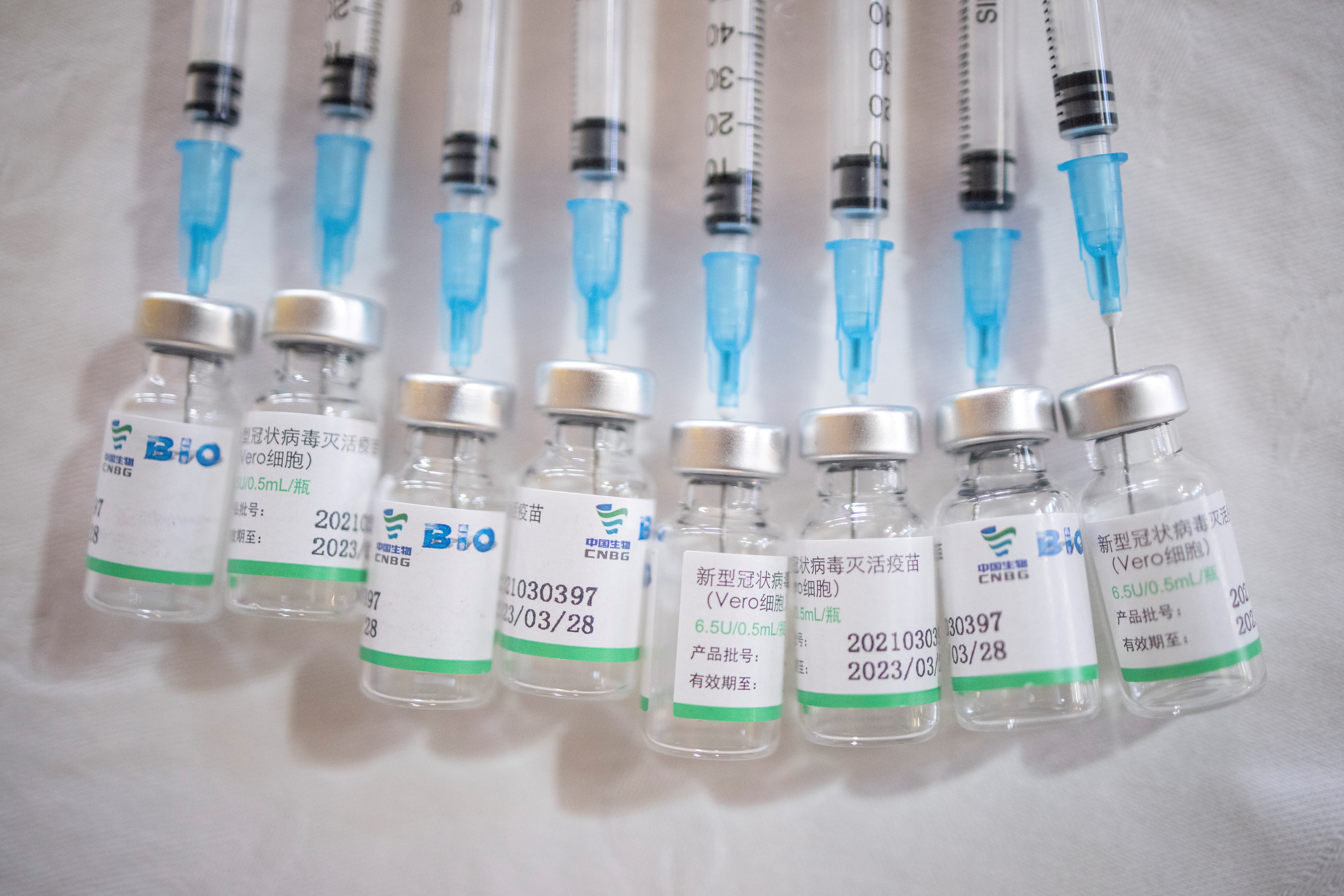
Sinopharm S Two Covid 19 Shots Effective Study Says Reuters

Uae Launches Covid 19 Vaccine Production With China S Sinopharm Reuters
Uae Rolls Out Sinopharm Vaccine To Children Aged 3 17 Dhaka Tribune

Chinese Citizens Are Already Receiving A Coronavirus Vaccine The New Yorker
The Uae Approves A Chinese Made Coronavirus Vaccine For Emergency Use

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
Chinese Covid 19 Vaccine Has 86 Efficacy Uae Says Coronavirus The Guardian
China Injects Hundreds Of Thousands With Experimental Covid 19 Vaccines Wsj

Why Who Approval Is Not Enough For Chinese Covid 19 Vaccines Coronavirus Outbreak News
China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says

Uae To Offer Third Chinese Vaccine Dose Amid Efficacy Concerns Coronavirus Pandemic News Al Jazeera
Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace
China S Vaccine Diplomacy Assumes Geopolitical Importance Merics

